Introduction
Radical prostatectomy remains a cornerstone in the management of localized prostate cancer. While surgical refinements and nerve-sparing approaches have significantly improved outcomes, erectile dysfunction (ED) continues to be one of the most prevalent and distressing sequelae. For many men, postoperative sexual dysfunction represents not just a physiological challenge but also an existential threat to identity, intimacy, and quality of life.
In this setting, the concept of penile rehabilitation has emerged. The rationale is simple: by promoting early penile oxygenation and regular erectile activity, one may preserve cavernosal smooth muscle, prevent fibrosis, and facilitate long-term recovery of spontaneous erections. Yet the implementation of rehabilitation is anything but straightforward. Protocols vary, evidence remains mixed, and patient adherence is often limited.
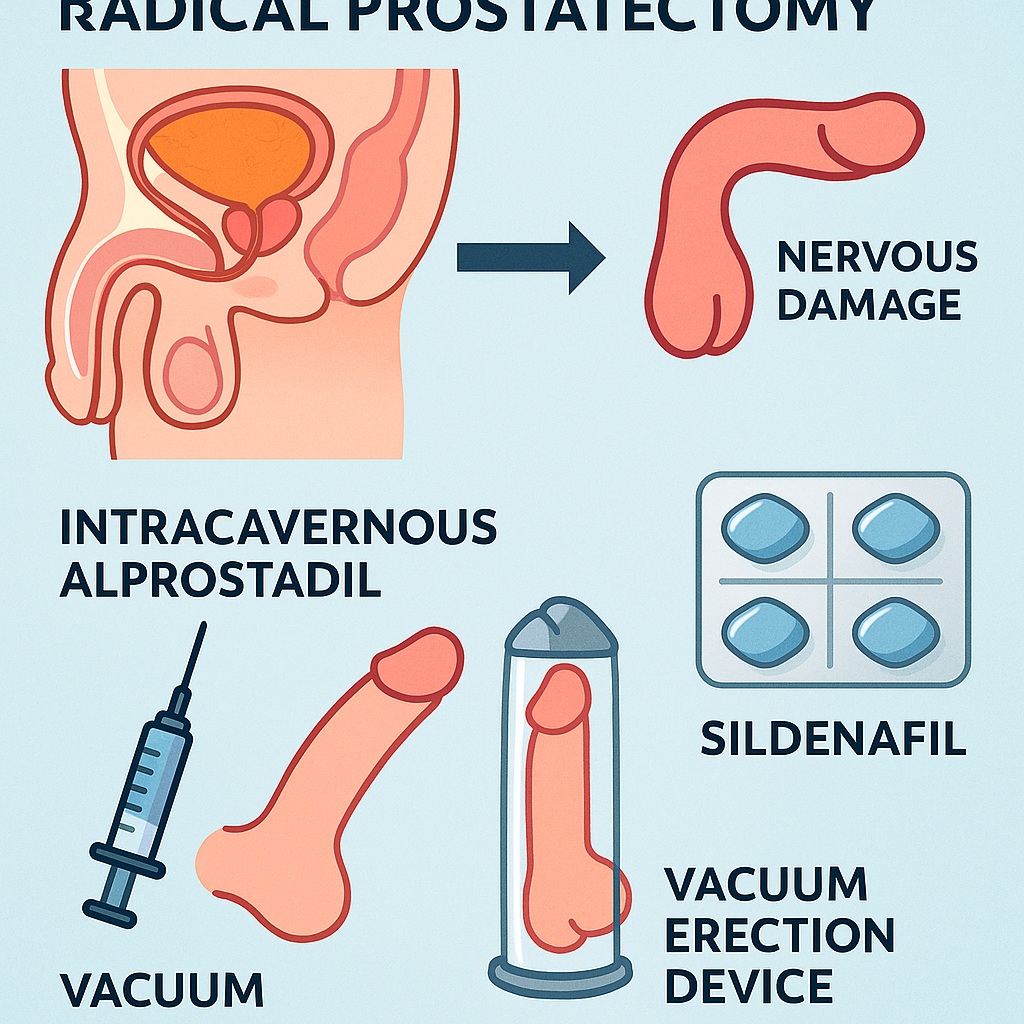
Among the various strategies, intracavernous alprostadil injections occupy a unique niche. Unlike oral phosphodiesterase type 5 inhibitors (PDE5i) such as sildenafil, which require some degree of intact neural input, alprostadil acts directly on penile tissue, bypassing the injured neurovascular pathways. This pharmacological property renders it particularly relevant in the early postoperative months, when nerve recovery is incomplete.
This article explores the biological rationale, clinical evidence, and therapeutic nuances of intracavernous alprostadil in sexual rehabilitation following radical prostatectomy. We also situate alprostadil within the broader rehabilitation landscape, considering its interplay with sildenafil, vacuum erection devices, and psychosocial interventions.
Pathophysiology of Post-Prostatectomy Erectile Dysfunction
Erectile function depends on a delicate interplay between neural, vascular, and smooth muscle components. Radical prostatectomy, despite nerve-sparing techniques, can disrupt this balance in multiple ways.
First, neuropraxia of cavernous nerves is nearly universal. Even without transection, traction and thermal injury lead to transient conduction block. During this period, neural input to penile tissue is diminished, impairing nitric oxide release and cGMP-mediated smooth muscle relaxation.
Second, cavernosal hypoxia ensues. Without regular nocturnal or spontaneous erections, oxygen delivery to penile tissue declines. Hypoxia triggers fibroblast activation, collagen deposition, and smooth muscle apoptosis. These structural changes reduce compliance of the corpora cavernosa, making future erections mechanically more difficult.
Third, psychological factors amplify physiological impairment. Anxiety, depression, and altered body image compound neural and vascular deficits, creating a vicious cycle.
Collectively, these mechanisms explain why early intervention is crucial. Penile rehabilitation aims to interrupt this cascade, preserving tissue health until natural recovery can occur.
Alprostadil: Pharmacology and Mechanism of Action
Alprostadil is a synthetic analogue of prostaglandin E1 (PGE1). When injected directly into the corpora cavernosa, it activates adenylate cyclase, increasing intracellular cAMP and causing smooth muscle relaxation. Unlike sildenafil, which potentiates endogenous nitric oxide signaling, alprostadil bypasses neuronal input entirely.
This distinction is clinically significant. In the early months post-prostatectomy, when neurogenic signaling is absent or reduced, sildenafil may fail to induce erections. Alprostadil, by contrast, remains effective. Furthermore, by improving cavernosal oxygenation, alprostadil may protect against fibrosis and structural deterioration.
Intracavernous administration ensures rapid onset and high efficacy. Success rates exceed 70–80% in well-instructed patients, making it one of the most reliable modalities for inducing erections post-surgery. However, barriers such as needle aversion, pain, and fear of priapism limit adherence.
Clinical Evidence for Alprostadil in Rehabilitation
Multiple clinical studies have evaluated intracavernous alprostadil as a rehabilitation tool. While methodologies differ, a consistent theme emerges: regular alprostadil use improves both short-term erectile activity and long-term functional outcomes.
In prospective cohorts, men receiving scheduled alprostadil injections from the early postoperative period demonstrate higher rates of spontaneous recovery compared to controls. Histological analyses of cavernosal biopsies confirm reduced fibrosis and preserved smooth muscle content in treated patients.
The study underpinning this review1-s2.0-S205011611530043X-mainreinforces these findings. Patients receiving structured intracavernous alprostadil rehabilitation reported improved erectile rigidity, greater satisfaction, and higher likelihood of resuming penetrative intercourse. Importantly, adherence correlated with outcomes, underscoring the necessity of patient education and support.
Randomized trials remain relatively small, and heterogeneity complicates meta-analyses. Nevertheless, the cumulative evidence supports alprostadil as a valuable component of multimodal rehabilitation strategies.
Comparing Alprostadil with Sildenafil and Other PDE5 Inhibitors
PDE5 inhibitors revolutionized ED management in the late 1990s, with sildenafil leading the charge. Their convenience, oral route, and favorable safety profile made them first-line therapy for most causes of ED. However, in the context of radical prostatectomy, their efficacy is variable.
Sildenafil requires intact neural signaling to facilitate nitric oxide release. In the setting of cavernous nerve neuropraxia, early use may yield disappointing results. Some studies suggest that chronic sildenafil administration may still confer rehabilitation benefits by enhancing endothelial function and increasing cavernosal oxygenation, even in the absence of erections. But as a practical erectile facilitator in the immediate postoperative months, sildenafil is often less effective than alprostadil.
By contrast, intracavernous alprostadil does not rely on nerve integrity. Its robust pharmacodynamic profile ensures reliable erections, making it particularly suited for early rehabilitation. Over time, as nerve recovery progresses, sildenafil and other PDE5i can be reintroduced, offering a less invasive long-term solution.
Thus, alprostadil and sildenafil should not be seen as competitors but as complementary tools—alprostadil for bridging the early postoperative gap, sildenafil for sustaining recovery in the long run.
Patient Selection and Counseling
Not every patient is an ideal candidate for intracavernous alprostadil. Success hinges on careful selection and thorough counseling.
Candidates should be motivated, cognitively intact, and free of significant manual dexterity limitations. Psychological readiness is equally crucial; needle aversion remains a major barrier. Training sessions, ideally supervised by a nurse specialist, help patients master injection techniques and build confidence.
Counseling must also address expectations. Rehabilitation is not an instant cure but a long-term investment in tissue preservation and functional recovery. Patients should be informed about side effects—penile pain, priapism, fibrosis at injection sites—and trained to recognize warning signs.
Partners play a pivotal role. Involving them in education fosters shared responsibility and may improve adherence. The relational dimension of rehabilitation should never be underestimated; sexual health is as much about intimacy as it is about hemodynamics.
Adherence and Real-World Challenges
Despite its efficacy, alprostadil suffers from adherence challenges. Dropout rates in studies often exceed 40–50% over the first year. Pain at injection, fear of complications, and psychological resistance contribute.
Strategies to improve adherence include:
- Stepwise dose titration to minimize pain and optimize efficacy.
- Regular follow-up visits to reinforce technique and address concerns.
- Integration with less invasive modalities, such as sildenafil or vacuum devices, to reduce dependence on injections.
Technology may assist. Smartphone reminders, telemedicine follow-ups, and digital instructional tools could enhance adherence and demystify the process. Rehabilitation is not merely a medical intervention but a behavioral journey requiring sustained support.
Beyond Alprostadil: The Future of Penile Rehabilitation
While alprostadil remains central, the future of rehabilitation is broader. PDE5 inhibitors continue to play a role, particularly as nerve recovery advances. Vacuum erection devices provide non-invasive oxygenation and mechanical preservation, albeit with limited spontaneity. Penile prostheses remain the definitive option for refractory cases.
Emerging therapies—low-intensity shockwave therapy, stem cell injections, platelet-rich plasma—are under investigation. Their promise lies in regenerative potential, offering not just symptom management but biological recovery. For now, these remain experimental.
An integrated approach, combining pharmacological, mechanical, and psychological interventions, is most likely to succeed. Rehabilitation must be personalized, accounting for surgical details, patient comorbidities, and psychosocial context.
Clinical Implications
The clinical message is clear: intracavernous alprostadil is a powerful tool for sexual rehabilitation after radical prostatectomy. Its ability to bypass neural pathways and directly oxygenate cavernosal tissue makes it uniquely suited for the early postoperative window.
When combined with agents like sildenafil, vacuum devices, and psychosocial support, alprostadil contributes to a holistic rehabilitation strategy. The goal is not simply to restore erections but to preserve identity, intimacy, and quality of life.
Urologists must therefore view rehabilitation not as an optional add-on but as an integral component of prostate cancer survivorship care.
Conclusion
Radical prostatectomy, while life-saving, often leaves men with the unintended consequence of erectile dysfunction. Intracavernous alprostadil injections, though invasive, represent one of the most effective strategies to preserve erectile tissue health and facilitate recovery.
By integrating alprostadil with sildenafil and other modalities, clinicians can offer a balanced, phased rehabilitation approach. The ultimate goal extends beyond erections—it is about restoring wholeness, dignity, and intimacy in the lives of prostate cancer survivors.
The challenge now lies in implementation: educating patients, supporting adherence, and ensuring access. If we succeed, sexual rehabilitation may become not just a clinical intervention but a standard of compassionate survivorship care.
FAQ
1. Why is erectile dysfunction common after radical prostatectomy?
Because surgery disrupts cavernous nerves, reduces penile oxygenation, and induces tissue remodeling, leading to impaired erections.
2. How does alprostadil differ from sildenafil?
Alprostadil works directly on penile smooth muscle, independent of neural signaling, making it effective even in early postoperative neuropraxia. Sildenafil requires intact neural pathways.
3. What are the risks of intracavernous injections?
Main risks include penile pain, fibrosis at injection sites, and rare priapism. Proper instruction and monitoring reduce these risks significantly.
4. Is penile rehabilitation truly effective?
Evidence suggests that structured rehabilitation improves both tissue health and functional recovery. Success depends on adherence, patient selection, and multimodal integration.
References
- Montorsi F, Brock G, Lee J, et al. Effect of nightly sildenafil on erectile function after bilateral nerve-sparing radical prostatectomy: a randomized, double-blind trial. J Urol. 2008;179(5):1478–1483.
- Padma-Nathan H, McCullough AR, Levine LA, et al. Randomized, double-blind, placebo-controlled study of postoperative nightly sildenafil for penile rehabilitation. J Urol. 2008;179(5):1465–1472.
- Mulhall JP, Bella AJ, Briganti A, McCullough A. Penile rehabilitation after radical prostatectomy: what are the facts, what are the myths? Urology. 2010;75(3):439–446.
- Bannowsky A, Schulze H, van der Horst C, Hautmann S, Junemann KP. Recovery of erectile function after nerve-sparing radical prostatectomy: improvement with nightly low-dose sildenafil. Eur Urol. 2008;54(2):282–288.
- Montorsi F, Guazzoni G, Strambi LF, et al. Recovery of spontaneous erectile function after nerve-sparing radical retropubic prostatectomy with and without early intracavernous injections of alprostadil. J Urol. 1997;158(4):1408–1410.
- Raina R, Pahlajani G, Agarwal A, Zippe C. Early use of intracavernous injections after radical prostatectomy improves erectile function recovery: results of a prospective study. Int J Impot Res. 2006;18(5):446–451.
- Montorsi F, Rigatti P, Carmignani G, et al. Alprostadil versus placebo in penile rehabilitation after radical prostatectomy: a multicenter randomized study. Eur Urol. 2004;45(5):650–656.
- Bella AJ, Mulhall JP. Penile rehabilitation following prostate cancer treatment: review of current literature. Can J Urol. 2012;19(3):6314–6320.